There is a new way to generate electricity from seawater

Scientists have used sunlight to turn seawater into hydrogen peroxide (H2O2), which can then be used in fuel cells to generate electricity
Scientists have used sunlight to turn seawater into hydrogen peroxide (H2O2), which can then be used in fuel cells to generate electricity
The solar energy sector is growing fast, despite the large fluctuations in the energy produced depending on the length of the daytime. This disadvantage could be overcome, however, when solar power is stored in the form of chemical energy and used as a fuel to produce electricity. A team of researchers, led by Shunichi Fukuzumi at Osaka University, has developed a method of photocatalytic production of hydrogen peroxide (H2O2) from seawater, which can subsequently be used to generate electricity in fuel cells. Their work was recently published in the journal Nature Communications.
Up to now, H2 -produced by photocatalytic pure water splitting- is typically used in fuel cells to generate electricity. However, H2 production has low solar energy conversion efficiency and due to its gaseous nature, it is difficult to store and transport. H2O2, on the other hand, can also be used as a fuel, even though previous methods of producing it required too much energy. The Osaka team managed to produce H2O2 as an aqueous solution from water and O2 in the air, which is much easier and safer to store and transport in higher densities, compared to highly compressed hydrogen gas. This efficient photocatalytic method involves a new photoelectrochemical cell developed to produce H2O2 when sunlight illuminates the photocatalyst, which then absorbs photons and initiates chemical reactions with the energy, resulting in H2O2. A test conducted for 24 hours shows that the H2O2 concentration in seawater reached about 48mM (millimolar), compared to 2mM in pure water. The researchers discovered that chlorine in seawater helped enhance the photocatalytic activity and led to the unusually high concentration of H2O2, and they expect that the method’s efficiency can be further improved by using better materials in the photoelectrochemical cell. "In the future, we plan to work on developing a method for the low-cost, large-scale production of H2O2 from seawater," Fukuzumi said. "This may replace the current high-cost production of H2O2 from H2 (from mainly natural gas) and O2".
Source: Phys.org
Source: Phys.org
Want to read more like this story?

World's most expensive places to build
Sep, 03, 2018 | NewsNew research by Turner & Townsend has revealed the world's most expensive places to build....

Power plants’ CO2 emissions are turned into stone in Iceland
Aug, 12, 2016 | NewsA new process of turning carbon emissions to solidified carbonate when pumped underground is under r...

This 3D-printed residential house cost just $10,000
Mar, 14, 2017 | NewsThe first ever 3D-printed residential home was built on-site in just 24 hours in the town of Stupino...

Study suggests the construction of 2 vast dams in the North Sea to protect Europe from floods
Feb, 20, 2020 | NewsAccording to scientists, as seawater level rises due to climate change, a plan to address flooding...

M 7.2 earthquake struck Haiti: More than 1,900 fatalities
Aug, 19, 2021 | NewsNearly 2,000 people have been reported dead after a M 7.2 earthquake hit Haiti. The seismic shock s...

Australian researchers have developed a solar paint that turns water vapor into hydrogen
Sep, 18, 2017 | NewsIt draws moisture from the air, splitting it into oxygen and hydrogen, the cleanest source of energy...

World-first commercial carbon-capture plant opens in Switzerland
Jul, 10, 2017 | NewsIts aim is to capture 1% of global CO2 emissions by 2025 Its aim is to capture 1% of global CO2 emi...
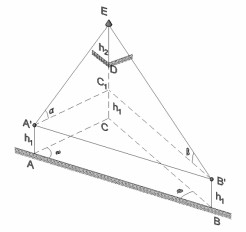
Calculation example - Calculate the height of an object when its base is inaccessible #2
Apr, 19, 2019 | EducationCalculate the height of an object from a baseline when its projected position to the ground is inacc...

China successfully tests new high-speed trains at top speeds of 453 km/h
Jul, 01, 2023 | NewsChina Railway announced on the first of July that successful performance tests had been conducted o...
Trending

Vertical gardens in Mexico City to combat pollution

Saudi Park Closed After 360 Big Pendulum Ride Crashes to Ground, 23 injured

Characteristics of Load Bearing Masonry Construction

Taipei 101’s impressive tuned mass damper

Dutch greenhouses have revolutionized modern farming

Federal court rules Biden’s offshore drilling ban unlawful


